Researchers at UC San Diego, along with others from the University of Southern California and Memorial Sloan Kettering Cancer Center in New York, have developed an innovative immunotherapy method to target and kill cancer cells remotely. This non-invasive method uses an ultrasound-based system to manipulate genetic processes in live immune T cells remotely, so they identify and kill cancer cells.
The study is published in the journal PNAS.
The researchers figured out how mechanogenetics could help them use ultrasound technology to disrupt the mechanical properties of immune T cells and convert their mechanical signals to genetically control the cells.
What is mechanogenetics?
Mechanogenetics is an emerging field of science that focuses on how physical forces and changes in the mechanical properties of cells and tissues influence gene expression so as to control gene and cell activations remotely.
By using this remote-controlled mechanogenetics process, the researchers were able to engineer chimeric antigen receptor (CAR)-expressing T cells capable of targeting and killing cancerous cells at tumor sites.
These engineered CAR-T cells have mechano-sensors and genetic transducing modules that can be remotely activated through the ultrasound process.
How does this process work?
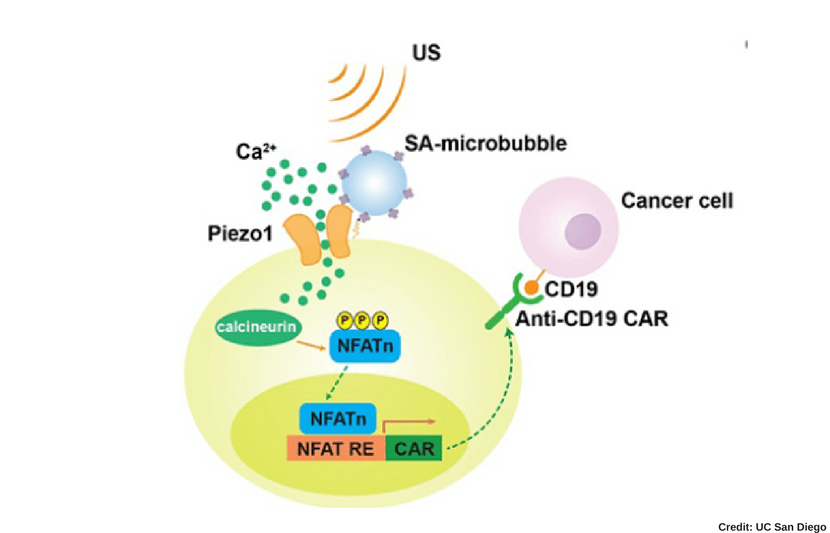
The process to remotely activate the CAR-T cells works through the ultrasound via microbubble amplification. In other words, this process takes place through a series of chemical responses to the ultrasound.
The microbubbles, combined with streptavidin, can be connected to the surface of the cell, where the mechanosensitive protein Piezo1 ion channels are expressed. Once the cell is exposed to ultrasound, the microbubbles vibrate and mechanically stimulate Piezo1 ion channels to open and allow calcium ions inside the cell.
This then triggers downstream pathways, including calcineurin activation, NFAT (nuclear factor of activated T cells) dephosphorylation, and translocation into the nucleus. Then the nucleus-translocated NFAT binds to upstream response elements of genetic transducing modules to initiate gene expression of CAR to recognize and kill target cancer cells.
CAR-T cell therapy has become a groundbreaking therapeutic approach for cancer treatment, though many challenges, such as the non-specific targeting of CAR-T cells against non-malignant tissue, have prevented it from becoming a widespread practice, Peter Yingxiao Wang, professor of bioengineering at UC San Diego, explained in a press release.
However, the team’s study “could ultimately lead to an unprecedented precision and efficiency in CAR-T cell immunotherapy against solid tumors, while minimizing off-tumor toxicities,” Wang said in a statement.
This is because “CAR-T cells will only be activated at tumor sites, hence avoiding non-specific actions against non malignant tissues,” Wang told The University Network (TUN).
The researchers are currently in the process of testing the technology on mice models, and will move to cancer patient trials next.
“This research should allow us to engineer remote-controllable cells of cells for therapeutics and clinical applications in precision medicine,” Wang told TUN.