We all learned about photosynthesis at some point in our education. We were taught that plants use sunlight to synthesize energy for their survival while creating oxygen for us to breathe.
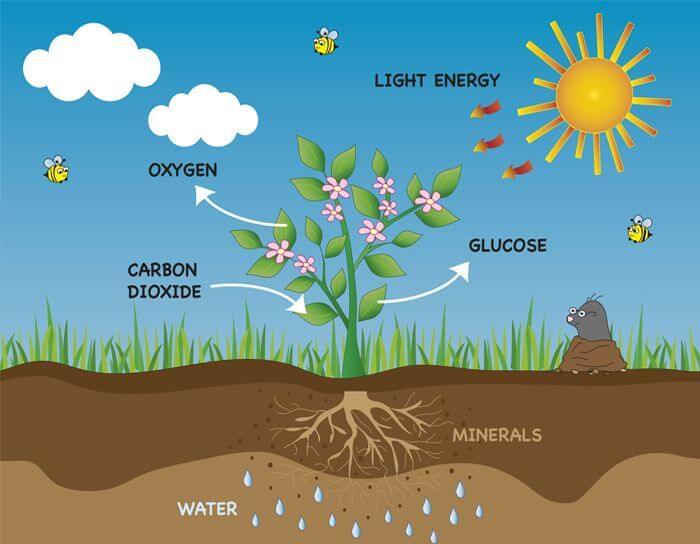
This process, which defines life as we know it, is simple to grasp.
But how do plants actually convert light into energy?
That’s always been a mystery until now, thanks to researchers from the University of Sheffield in the UK who have just unlocked the secrets of photosynthesis and successfully used the underlying mechanics to “direct energy transfer via light at a molecular level.” The research is groundbreaking, and paves the way for future inventions that rely upon the transfer of energy through light, including solar and computing technology.
The university’s research team is led by Professor Julia A. Weinstein, a professor of physical chemistry, and Dr. Anthony J.H.M. Meijer, a reader in theoretical chemistry.
The research paper is published in Nature Chemistry.
The research
Photosynthesis, in simple terms, is the process by which plants capture sunlight, using chlorophyll, and mix it with water, carbon dioxide, and minerals to produce food, which they use for energy, and oxygen, which they breathe out for us to breathe in.
Photosynthesis thus gives plants the ability to create and store energy, which involves the transfer of electrons. This “energy and charge transfer” also forms the basis for conversion of solar energy to chemical energy, or of electrical to chemical energy.

The ability to “switch electron transfer on or off” is not new, according to Weinstein.
“What makes our research so exciting is that, via our synthetic molecule, we can now direct the path of an electron in a very specific and controlled way,” Weinstein said in a statement.
Weinstein is referring to a new “fork” molecule used by the researchers, which “can direct the destination of an electron in a precise manner when a particular infrared light pulse is applied.”
“In creating this ‘molecular fork’, we now have the ability to model natural molecular processes, such as photosynthesis,” Weinstein said in a statement.
“If we can replicate how energy is stored and utilised, then we have the basis to develop exciting new molecular technologies for the future.”
The researchers believe that the ability to direct energy along one of several pathways by way of molecular forks has many potential applications.
“From new ways of capturing and storing the energy coming to us from the Sun [sic], to developing new forms of computing technology, this research opens up some exciting new opportunities,” said Weinstein in a statement. Examples provided by the researchers included information storage and retrieval in computing, where the molecular fork could be used to direct charge, “using low-energy red light.”
The research was funded by the Engineering and Physical Sciences Research Council (EPSRC) and Science and Technology Facilities Council (STFC).
Further details of the research
While photosynthesis is a simple concept, the research is complex. In an effort to capture the complexity of the study, The University Network (TUN) set out a Q&A with Weinstein and Meijer to find out, in their own words, what was involved in their research.
How does energy and charge transfer drive photosynthesis and any solar-to-chemical or electrical-to-chemical energy conversion?
When a photon strikes a molecule, it can be absorbed and the molecule becomes in an “electronically excited”, higher energy state. It means that electron density distribution in the molecule has changed – some regions become more electron-rich, and some less electron-rich.
In photosynthesis, this shift of electron density is causing “charge separation” – electron is promoted from one part of the assembly to another, leaving behind a +-vely charged vacancy, which is usually termed a hole.
In effect, the energy of the absorbed photon has been spent on separating a “-” and a “+” charge, and the energy is now stored in this higher-energy, charge-separated state. The part of the molecule that loses the electron is called a donor, and the one which takes the electron – an acceptor.
Normally, electron and hole recombine and there is no net effect of the photon hitting the molecule. However, if one spatially separates electron and hole, i.e., if one moves the hole to one end of the molecule and the electron to the other end, then the recombination can be slowed down, and these charges can persist for long enough to be used – either harvested as electricity in photovoltaics, or drive some useful chemistry. This is the key driver for photosynthesis or any solar-electrical-chemical energy conversion.
In the case of photosynthesis, the electrons are used to reduce CO2 and ultimately make glucose. At the same time, the holes are used to oxidise water and release oxygen. The spatially separated electron and hole could also be used directly, since moving electrons are of course electricity (that is how solar panels work in a nutshell).
Getting the electron to the right place to perform the reduction of CO2 in photosynthesis is less easy than it sounds, and there are indications that subtle interactions (quantum interference) between the electronic states of different parts of the light harvesting protein play a crucial role. Such interferences are not dissimilar to the one we used in this paper to get the outcome we wanted.
How did you create the ‘molecular fork’?
The molecular fork idea is coming from the multitude of electronically similar if not identical pathways in photosynthesis. We wanted to see if we could channel charges in one out of many available pathways in an artificial system. But how to make the two “roads” identical yet distinct?
The key behind the molecular fork is that the two arms contain the same elements overall, but different isotopes of carbon for the acetylide units, which bridge two donors to one acceptor. So, the two arms are chemically the same, but vibrate with different frequencies. That means that we can “target” these vibrations individually, affecting one arm but not another. This involved a really hard piece of synthesis.
Please tell us more about the exciting opportunities opened up by your research.
Whilst inspired by photosynthesis, this is fundamental research, which is yet to evolve into direct applications. However, this is the first time this effect has been demonstrated and there are a number of areas where this could become really exciting.
First of all, our setup allows to direct charges down one or the other arm, at will. So, we are effectively switching the path of the hole between one of the two possible paths it can take. This makes the platinum-acetylide bridge part of the complex effectively a photo switch (or a set of points, if you want use a railway analogy). (Fast) switching is key to all our modern technology, but of course the internet in particular. Our molecule is a molecular version of the kind of switches, which are ubiquitous in letting computers talk to each other and could therefore offer opportunities in this area. Photo switches have great potential in information storage and retrieval.
What’s more, we use a low-energy infrared light – which carries <10% energy of a photon in the visible range – to alter the reactivity path taken by the molecule. The infrared light does not affect anything else in the molecule, it is really targeting, specifically, one and only vibration that we want to target.
Overall, a quantum of low-energy infrared light can “tell” the molecule where to send charges – “left or right.” That could be used in logic gates, information storage, reactivity-at-will…anything that needs a “switch” and relies on more than one potential outcome. One can see this as “molecular choice.”
Conclusion
This groundbreaking research should pave the way for advances in technology in many sectors.
“This is a really exciting area of research inspired by photosynthesis; it uses the latest advances in both modern technology of ultrafast, tuneable, short-pulse lasers and theoretical approaches to quantum mechanics in large systems, in an attempt to direct reactivity at will,” Weinstein and Meijer told TUN.
“The ability to control light-induced reactivity, light-induced action and function, is immensely exciting, and we think much of the future in nanotechnology and computer technology will have the prefix ‘photo’ – when photocontrol of the nanoworld takes off.”
The researchers credit their success to their collaboration with others.
“This cross-disciplinary work was brought about by an amazingly lucky combination of extremely talented synthetic chemists, laser spectroscopists, and theoreticians, from the University of Sheffield and Central Laser Facility at Rutherford Appleton Laboratory – a sentence that says that the interdisciplinary research is the key!,” Weinstein and Meijer told TUN.